She then capped the clear solution and set it aside on the lab. This is observed that these solutions of.

Solved Which Of The Statements Below Best Describes The Chegg Com
Calculate the new molarity if each of the following dilutions is made.
. A technican prepared a solution by heating 100 mL of distilled water while adding KCl crystals until no more KCl would dissolve. The hydrogen-releasing reaction is why potassium metal is so dangerous around water or moisture. After the solid is.
H1 as the only positive ion in solution. Assume the volumes are additive. KOHs K aq OHaq C.
Each of these ions are then completely solvated ie. HCl aq KOH aq KCl aq H₂O l The letters aq in parenthesis next to the chemical formula of a compound is used to indicate aqueous phase. O KOH s H20 1 - KO aq H30 ag.
NaOH its suspension dissolves. A 523 mL of water is added to 271 mL of 0124 M KOH solution b. 41Which of the following reactions best describes.
Which of the following reactions best describes the dissolution of solid KOHs in water. KOH CH3OH CH3OK H2O. Question 16 of 25 40 40 Points If you have a giant stockpile of 500 M KOH and you pour out some of it into a 750 mL container how many moles did you pour out.
NH4 as the only positive ion in solution. It means that the. To prepare 1000 mL of a 01 molL solution of Potassium hydroxide we have to dissolve 56105 g of KOH 100 purity in deionized or distilled water.
2K 2H2O - 2KOH H2. When an Arrhenius acid is dissolved in water it produces. Potassium hydroxide is a basic oxide which all dissolve in water to form base solutionsPotassium hydroxide is actually the product of reacting potassium metal with water.
In the case of methanol the potassium methoxide methylate forms. To prepare 60 solution weight by volume basis of a salt you have to dissolve 60 g salt in 100 ml water. QUESTION 6 Which of the following reactions best describes the dissolution of solid KOH s in water.
KOH completely dissociates in water to its constituent ions - K and OH-. KOHs KO aq H aq B. If you drop a 50 gram piece of metal that has a temperature of 110Celsius into 1000 grams of water at 25Celsius what best describes what would occur.
KOHs - KOHaq D. A 555g sample of a weak acid with ka1310-4 was combined with 500ml of 600. Surrounded by water molecules so that the charges on the ions.
Chemistry questions and answers. The best description of salt is that when they are dissolved in water they dissociate into ions and become electrolytic in nature. Which below best describes the behavior of an amphoteric hydroxide in water.
Alcoholic KOH dissociates in water to give RO- ions which is a strong base. CHEM1411 Which of the following reactions best describes the dissolution of solid KOHS in water. It abstracts hydrogen giving rise to elimination in reaction.
HCl its suspension dissolves. Questions Courses 41Which of the following reactions best describes the dissolution of solid KOH s in water. If 200 mL of a 0500 M HCl.
Because of its high affinity for water KOH serves as a desiccant in the laboratory. Calculate the menthyl acetate content in if 95 g sample of peppermint oil was. HNO3 is a strong base and KNO3 is the salt of a strong base KOH and a.
But to prepare solution of a given molarity say to prepare 1 M KOH solution you. KOHs Kaq OH-aq Which of the following chemical equations best represents the. We generally use alcoholic KOH to.
Which of the following reactions best describes the dissolution of solid NaOH s in water.
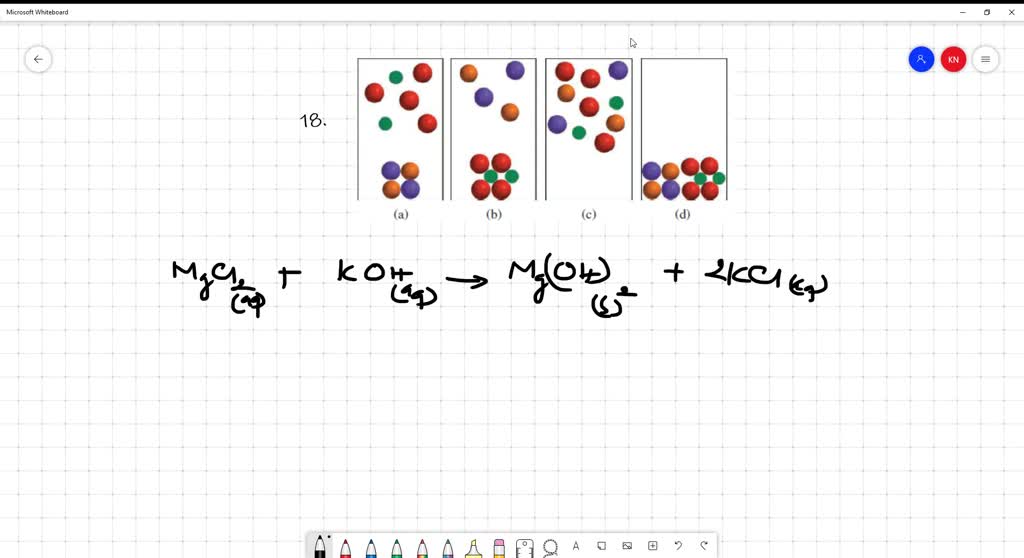
Solved Two Aqueous Solutions Of Mathrm Koh And Mathrm Mgcl 2 Are Mixed Which Of The Following Diagrams Best Represents The Mixture For Simplicity Water Molecules Are Not Shown Color Codes Are K Purple Left Mathrm Oh Mathrm Red

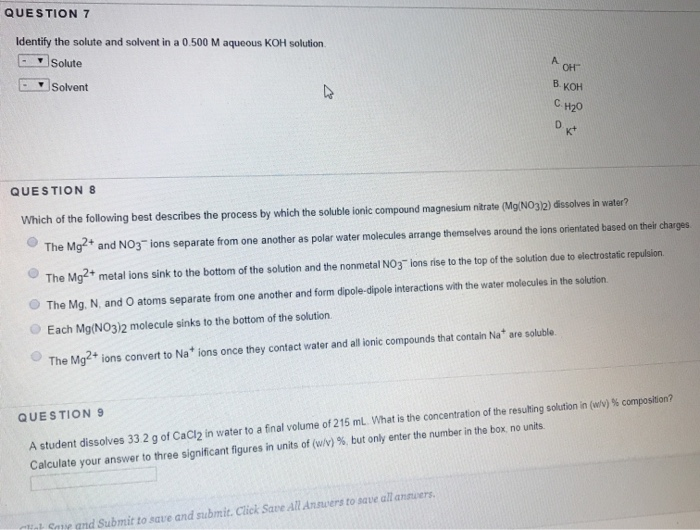
Solved Question 7 Identify The Solute And Solvent In A 0 500 Chegg Com

Solved Chem1411 Which Of The Following Reactions Best Chegg Com
0 Comments